NANA – Registry-based non-randomized studies for assessing the benefits and harms of drugs
Department of Medical Statistics
Overview
The research team in the NANA project is investigating whether it is currently possible and sensible to use the registry data available in Germany for assessing the benefits and harms of new drugs.
For this purpose, the team is carrying out ten to twelve register-based non-randomized studies. The results of these analyses will be compared with the existing results on the same clinical question from randomised controlled trials.
The project aims to provide insights into the circumstances under which an analysis based on routinely collected data can provide reliable results on the benefits and harms of drugs and which aspects need to be carefully considered when such analyses are implemented.
The project is funded by the “Federal Joint Committee”. Find here the project description of the “Innovationsausschuss”.

Project process
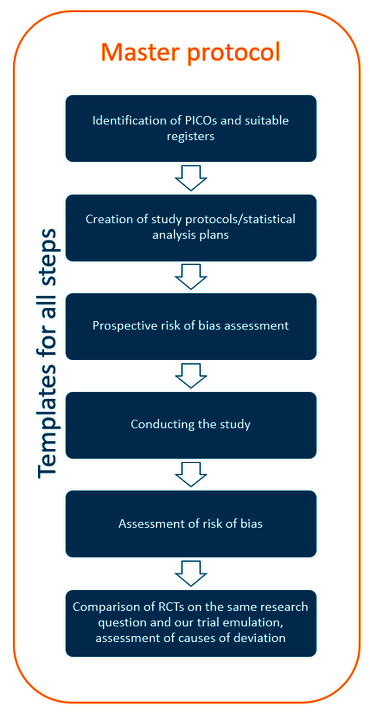
Starting point of each emulation was a clinically relevant question formulated under the PICO framework and identified to equal parts through one of two possible processes. Our advisory board consisting of pharmacological experts compiled a list of currently relevant questions for which we tried to identify and recruit suitable registers.
In addition, we contacted potentially suitable German registers and recruited those that were interested and offered to provide suitable data. Questions were then formulated by clinical experts chosen by cooperating registers.
The basic PICOS are translated to more detailed target trials through iterative discussions with the clinical experts. If an emulation is feasible in principle using the available data, we create a study protocol and conduct a prospective risk of bias assessment.
While we conducteach non-randomized registry-based cohort study, we record all feasibility problems and relevant methodological aspects.
This is followed by a final bias assessment and a systematic comparison of randomized controlled trials (RCTs) on the same clinical question (if available) and our results in order to assess causes of deviation between the two.
Besides gaining insights on the feasibility of conducting such non-randomized studies using currently available German registry data we also aim to support the dissemination of state-of-the art statistical methodology.
We develop materials like templates for all important steps of the analysis process and reusable R analysis scripts that will be made available to the public.
Given talks and presentations
As part of the NANA project, the following presentations and talks were given:
- Feasibility of Benefit-Risk Evaluation of Drugs based on Non-Randomized Studies using German Registries: First Results from the NANA Project
by Paula Starke, Enes Malik Cakir, Prof. Dr. Tim Friede, Prof. Tim Mathes (Department of Medical Statistics, UMG, Göttingen), held at “7th Joint Statistical Meeting der Deutschen Arbeitsgemeinschaft Statistik” - Target-Trial-Emulation mittels Registerdaten zur Nutzenbewertung von Arzneimitteln: Methodik, Herausforderungen und erste Erkenntnisse aus dem NANA-Projekt
by Paula Starke, Prof. Dr. Tim Mathes (Department of Medical Statistics, UMG, Göttingen), held at “Registertage 2025” - Registerbasierte Studien als Evidenz in der Arzneimittelbewertung?
by Paula Starke, Prof. Dr. Tim Mathes (Department of Medical Statistics, UMG, Göttingen), held at “3. Konferenz für registerbasierte Forschung des DNVF” - Einblick in das Projekt NANA
by Maxi Schulz (Department of Medical Statistics, UMG, Göttingen), held at “Deutscher Kongress für Versorgungsforschung, REGINT-Symposium” - Trial-Emulation mit Registerdaten
by Enes Malik Cakir, Paula Starke, Maxi Schulz (Department of Medical Statistics, UMG, Göttingen), held at "2. Jahrestagung der Deutschen Gesellschaft für pädiatrische und adoleszente Endokrinologie und Diabetologie"
Contact

Kontaktinformationen
- Telefon: +49 551 3961197
- Telefax: +49 551 3965605
- E-Mail-Adresse: enes.cakir(at)med.uni-goettingen.de
- Ort / Raum: Humboldtallee 32, EG 0.136

Kontaktinformationen
- Telefon: +49 551 3963021
- Telefax: +49 551 3965605
- E-Mail-Adresse: reginasharon.kampo(at)med.uni-goettingen.de
- Ort / Raum: Humboldtallee 32, E1. Stock, 1.127

Kontaktinformationen
- Telefon: +49 551 3961183
- Telefax: +49 551 3965605
- E-Mail-Adresse: susanna.salem(at)med.uni-goettingen.de
- Ort / Raum: Humboldtallee 32, 1. OG, 1.131

Kontaktinformationen
- Telefon: +49 551 3964982
- Telefax: +49 551 3965605
- E-Mail-Adresse: maxi.schulz(at)med.uni-goettingen.de
- Ort / Raum: Humboldtallee 32, EG, 140

Kontaktinformationen
- Telefon: +49 551 3964992
- Telefax: +49 551 3965605
- E-Mail-Adresse: paula.starke(at)med.uni-goettingen.de
- Ort / Raum: Humboldtallee 32, EG, 140
